Bioequivalence and bioavailability
Bioequivalence is defined as the interchangeability of two pharmaceutical specialties that have the same active principle ingredient (API) and equivalent bioavailability. This definition leads us to bioavailability, which refers to the amount of drug that actively reaches the circulation and the speed at which it accesses it, that is, the fraction of drug capable of reaching the site of action. It depends on the physicochemical characteristics of the active principle ingredient, the excipients, the manufacturing process and the preservation of the pharmaceutical form, and also on the intrinsic characteristics of the individual, such as intestinal motility or gastric pH.
Any modification in any of these factors can alter both the total amount of active principle ingredient absorbed and the rate at which it is absorbed. For this reason, in the development of a generic pharmaceutical specialty, despite the fact that it is the same active principle ingredient, the same dose and the same pharmaceutical form, these factors can determine a different bioavailability than that of the reference product.
Consequently, two pharmaceutical specialties have an equivalent bioavailability if both the concentration of the active drug and the rate at which it accesses the systemic circulation are within an interval considered equivalent, when they are administered at the same doses and under the same experimental conditions.

Bioequivalence studies
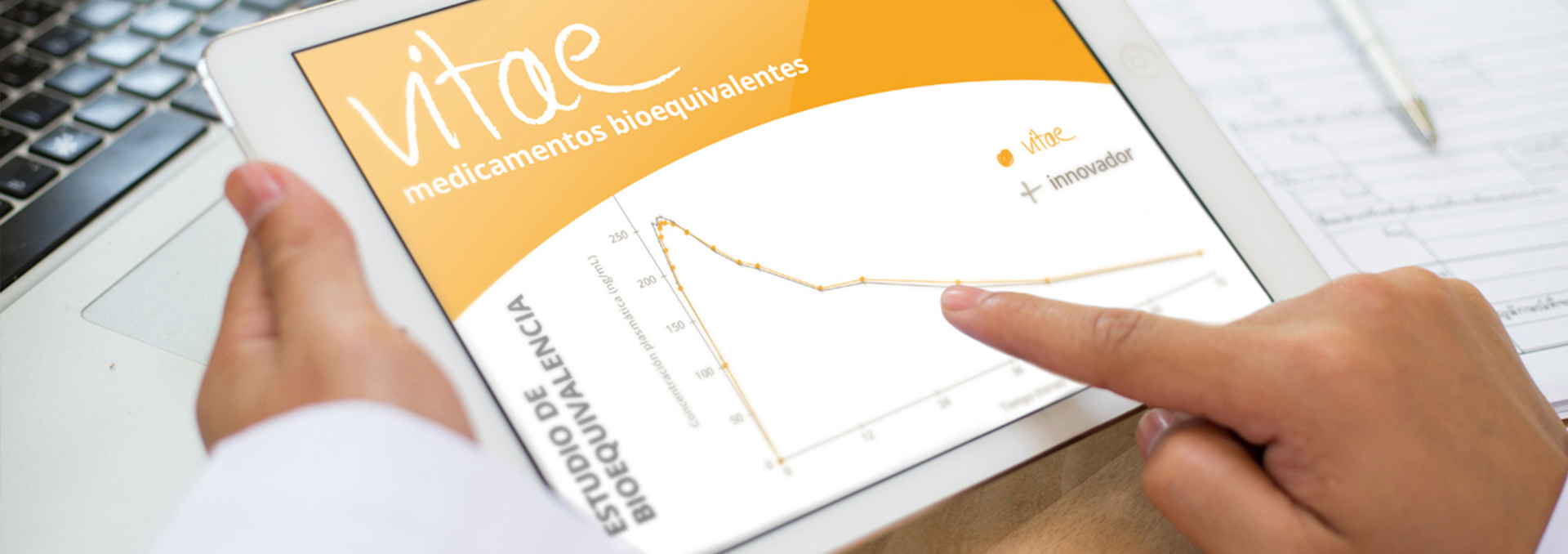
Bioavailability studies demonstrate the bioequivalence between two pharmaceutical specialties. These studies are carried out through controlled clinical trials, usually double-blind and crossover, in groups of 12 to 36 healthy volunteers assigned randomly, meeting the requirements defined in the Directive on Bioavailability and Bioequivalence Research of the European Agency for the Evaluation of Medicines. These requirements coincide with those required by the United States.
Pharmacokinetic parameters
The pharmacokinetic parameters required to determine the bioavailability of a pharmaceutical specialty are:
- The time when Cmax is reached (Tmax)
- The maximum plasma concentration (Cmax)
- The Area Under the Curve of plasma concentration over time (ABC or AUC in Anglo-Saxon terminology), which quantifies the total amount of active principle ingredient absorbed.

What guarantees does bioequivalence offer?
When the bioequivalence between a generic pharmaceutical specialty and the innovative medicine is issued, the healthcare administration guarantees that the patient's response (clinical efficacy) to both specialties will be similar.
The bioequivalence between a generic pharmaceutical specialty and the innovative medicine, guarantees to all healthcare professionals that their effects in terms of efficacy and safety will be essentially similar, and consequently the two specialties will be interchangeable. That is, one of them can replace the other in the treatment of a disease or a symptom in the patient.
Garantías Clave